|
Cyclin Dependent Kinase 2 (CDK2) Targeted Library |
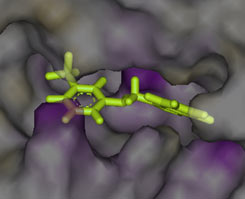 |
Ligand in CDK2 ATP-binding site.
Docking example from OTAVAchemicals CDK2 targeted library |
The cell division cycle is controlled by cyclin-dependent kinases (cdk), which consist of a catalytic subunit (cdk1-cdk8) and a regulatory subunit (cyclin A-cyclin H). These proteins are regulated in several ways: subunit production, complex formation, (de)phosphorylation, cellular localization, and interaction with various natural protein inhibitors.
Cyclin-dependent kinase 2 (CDK2) plays a critical role in the G1- to S-phase checkpoint of the cell cycle. Like several other protein kinases, CDK2 consists of a relatively small N-terminal domain that is rich in B-sheet structure and a larger C-terminal domain which is predominantly A-helical. The ATP-binding site is located in a pocket between the two domains.
Inhibition of cyclin-dependent kinases is a theme of major interest in current anti-cancer agents research. Different classes of chemical inhibitors of these enzymes have been identified during the past decade and the structural basis of inhibition has been elucidated by X-ray crystallography studies of one member of the family, CDK2. Several types of cdk inhibitors have so far been described: staurosporine, flavopiridole, butyrolactone purine derivatives, indirubin, paullones, and others.
The designed CDK2 Targeted Library comprises only drug-like compounds (PAINS compounds is filtered off). It provides a perfect basis for drug discovery projects related with cancer.
All compounds are in stock, cherry-picking is available.
The CDK2 Targeted Library (DB, SD, XLS, PDF format) as well as the price-list is available on request. Feel free to contact us or use on-line form below to send an inquiry if you are interested to obtain this library or if you need more information.
|
 HOME
HOME ABOUT
ABOUT
 SERVICES
SERVICES
 PRODUCTS
PRODUCTS
 Targeted Libraries
Targeted Libraries
 Biochemicals
Biochemicals
 RESEARCH
RESEARCH
 DOWNLOADS
DOWNLOADS ORDERING
ORDERING
 CONTACTS
CONTACTS

